Multiple Choice
Which statement about the phase diagram below is not correct? 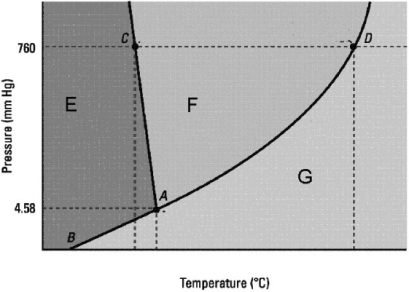
A) The critical point is not shown.
B) D is the normal boiling point.
C) The vapor pressure of the solid is zero below the triple point.
D) The normal melting point is at a lower temperature than the triple point.
E) E, F, and G label in that order the solid, liquid, and gas phases.
Correct Answer:

Verified
Correct Answer:
Verified
Q46: Given the van der Waals a constant
Q48: Which alcohol should be most soluble in
Q72: The relative energies (strengths) of the intermolecular
Q76: Henry's law constant for oxygen dissolving in
Q78: The relative energies (strengths) of the intermolecular
Q79: The relative energies (strengths) of the intermolecular
Q81: Point d in the phase diagram below
Q82: Which of the following diagrams best shows
Q110: Given the van der Waals a constant
Q141: Which of the following pairs of compounds