Multiple Choice
Boron nitride is being investigated in frontier research directed at producing novel electronic devices. If you used the following energy level diagram for the molecular orbitals of boron nitride, BN, what would you predict? 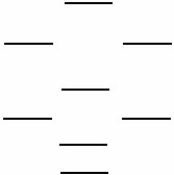
(I) Boron nitride is diamagnetic.
(II) Boron nitride has a bond order of 2.
(III) Boron nitride is paramagnetic.
(IV) The bond in BN- is stronger than the bond in BN.
A) I and II
B) II and III
C) I, II, and IV
D) III
E) IV
Correct Answer:

Verified
Correct Answer:
Verified
Q30: What is the hybridization of the bromine
Q56: What type of hybridization is needed to
Q57: Which of the following diagrams shows the
Q60: For the molecule CH<sub>3</sub>CHCHCH<sub>3</sub>, the local molecular
Q63: Which statement regarding a pi bond between
Q64: Identify the hybridization of the atomic orbitals
Q66: Identify the hybridization of atomic orbitals for
Q125: Which electron-pair geometry has the lowest electron-electron
Q136: Which statement regarding a sigma bond between
Q182: Draw a structure showing the geometry of