Essay
Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below. Explain why you think this molecule is planar or nonplanar. 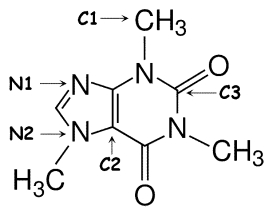
Correct Answer:

Verified
N1: sp2; N2: sp3; C1: sp3; C2 and...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q12: Which of the following ions is linear?<br>A)
Q51: Which of the following has the largest
Q61: Boron nitride is being investigated in frontier
Q62: What is the geometry of the ClF<sub>4</sub><sup>-</sup>
Q63: Which statement regarding a pi bond between
Q64: Identify the hybridization of the atomic orbitals
Q67: Which of the following compounds has a
Q120: Which type of molecular orbital contains a
Q125: Which electron-pair geometry has the lowest electron-electron
Q182: Draw a structure showing the geometry of