Short Answer
Identify the hybridization of atomic orbitals for atoms a, b, and c in the structure below of acetaminophen, which is the active ingredient in the analgesic Tylenol. 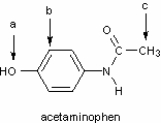
Correct Answer:

Verified
Correct Answer:
Verified
Q18: Which of the following molecules is paramagnetic?<br>A)Li<sub>2</sub><br>B)C<sub>2</sub><br>C)B<sub>2</sub><br>D)N<sub>2</sub><br>E)F<sub>2</sub>
Q30: What is the hybridization of the bromine
Q48: A Lewis structure of aspirin without the
Q51: Which of the following molecules is T-shaped?<br>A)
Q52: Which of the following compounds has the
Q56: What type of hybridization is needed to
Q57: Which of the following diagrams shows the
Q65: Use energy levels of diatomic molecules derived
Q71: Both cyclohexane (C<sub>6</sub>H<sub>12</sub>) and benzene (C<sub>6</sub>H<sub>6</sub>) have
Q77: Use MO theory to predict the bond