Essay
A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below. Taking into account the nonbonding electrons, identify the hybridization of the atomic orbitals for the following atoms: O1, O2, and O3. Identify the bond angles around C1, C2, and O3. 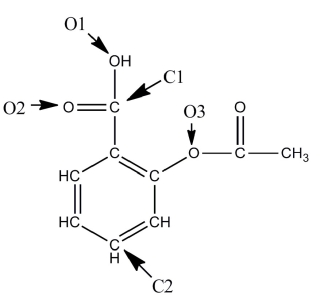
Correct Answer:

Verified
O1 is sp3 hybridized, O2 is sp2,...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q9: For the molecule CH<sub>3</sub>CHCHCH<sub>3</sub>, the local molecular
Q44: Which of these molecules have a dipole
Q46: Identify the molecular structure of the molecular
Q47: The amide structure is the fundamental linking
Q51: Which of the following molecules is T-shaped?<br>A)
Q52: Which of the following compounds has the
Q53: Identify the hybridization of atomic orbitals for
Q65: Use energy levels of diatomic molecules derived
Q77: Use MO theory to predict the bond
Q183: Which type of molecular orbital is used