Essay
The amide structure is the fundamental linking unit in proteins. Two partial Lewis structures for formamide are shown below. These structures do not show the lone pair electrons or the formal charges. Experiment shows that formamide is planar, so which is the better representation of the electronic structure, I or II? Is this conclusion consistent with the formal charges on the atoms? Explain the rationale for your answers. 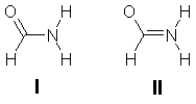
Correct Answer:

Verified
Structure II predicts a planar structure...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q9: For the molecule CH<sub>3</sub>CHCHCH<sub>3</sub>, the local molecular
Q44: Which of these molecules have a dipole
Q46: Identify the molecular structure of the molecular
Q48: A Lewis structure of aspirin without the
Q51: Which of the following molecules is T-shaped?<br>A)
Q52: Which of the following compounds has the
Q65: Use energy levels of diatomic molecules derived
Q77: Use MO theory to predict the bond
Q178: Carbonyl dihalides (COX<sub>2</sub> with X = I,
Q183: Which type of molecular orbital is used