Multiple Choice
The combustion of butane (C4H10) forms carbon dioxide and water. What is the stoichiometric coefficient for oxygen in the balanced equation when 1 mol of butane undergoes combustion?
A) 9
B) 13
C) 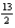
D) 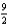
E) 5
Correct Answer:

Verified
Correct Answer:
Verified
Q15: A mass of 11.60 g of phosphoric
Q17: A bottle containing 1665 g of sulfuric
Q20: Hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>) decomposes catalytically in the
Q22: Hydrogen peroxide decomposes to produce water and
Q59: Which statement about the following chemical
Q60: The combustion of ethanol (CH<sub>3</sub>CH<sub>2</sub>OH, 46.1 g/mol)
Q67: Metallic copper can be obtained from the
Q79: Baking soda (NaHCO<sub>3</sub>, 84.0 g/mol) requires
Q96: Socrates was killed by a dose of
Q105: If the combustion of fossil fuels adds