Multiple Choice
Hydrogen peroxide (H2O2) decomposes catalytically in the presence of metal ions and enzymes called peroxidases. You may have noticed this reaction occurring if you use a drugstore solution of hydrogen peroxide as a mouthwash or antiseptic. If a 250 mL bottle of hydrogen peroxide solution containing 3.0% H2O2 (by mass) completely decomposes, how many liters of oxygen gas would it generate? Assume that 1 mol of gas occupies a volume of 22.4 L and that the density of the solution is 1.0 g/mL. The reaction is 2H2O2(aq) 2H2O( 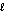 ) + O2(g)
) + O2(g)
A) 2.5 L
B) 4.9 L
C) 9.9 L
D) 7.4 L
E) 0.22 L
Correct Answer:

Verified
Correct Answer:
Verified
Q15: A mass of 11.60 g of phosphoric
Q17: A bottle containing 1665 g of sulfuric
Q19: The combustion of butane (C<sub>4</sub>H<sub>10</sub>) forms carbon
Q22: Hydrogen peroxide decomposes to produce water and
Q39: Phthalocyanine is a large molecule used in
Q59: Which statement about the following chemical
Q60: The combustion of ethanol (CH<sub>3</sub>CH<sub>2</sub>OH, 46.1 g/mol)
Q67: Metallic copper can be obtained from the
Q79: Baking soda (NaHCO<sub>3</sub>, 84.0 g/mol) requires
Q105: If the combustion of fossil fuels adds