Multiple Choice
According to the phase diagram given, which of the following is INCORRECT? 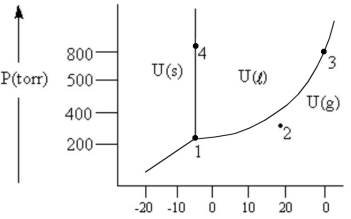
A) At the temperature and pressure of point 1, substance U exists as a three-phase equilibrium system.
B) At the temperature and pressure of point 2, substance U exists as a one-phase gaseous system.
C) At the temperature and pressure of point 3, substance U exists as a two-phase system.
D) If the U(s) ⇔ U(l) system is maintained at the temperature of point 4 while pressure is decreased steadily to about 300 torr, more U will freeze.
E) There are no conditions of temperature and pressure under which solid U will vaporize without melting first.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Given the following information, calculate ΔH°(in kcal
Q25: Given the following information, calculate ΔH°(in kcal
Q26: All sides are equal in length and
Q28: Vaporization occurs more readily with:<br>A) increased temperature,
Q30: CH<sub>4</sub> probably has a lower boiling point
Q32: Arrange in order by decreasing boiling point:
Q37: A liquid has a molar heat of
Q50: The enthalpy of condensation is equal to,but
Q97: If one compares compound A,composed of nonpolar
Q114: A liquid is in equilibrium with its