Multiple Choice
Given the following information, calculate ΔH°(in kcal mol-1) for: I(g) + e- → I-(g) 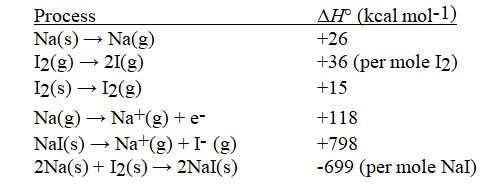
A) -633 kcal mol-1
B) -450 kcal mol-1
C) -71 kcal mol-1
D) -696 kcal mol-1
E) +712 kcal mol-1
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Given the following information, calculate ΔH°(in kcal
Q20: Below are given the Lewis structures of
Q22: If 34 g of a solid of
Q23: A liquid has a normal boiling point
Q25: Given the following information, calculate ΔH°(in kcal
Q26: All sides are equal in length and
Q28: Vaporization occurs more readily with:<br>A) increased temperature,
Q29: According to the phase diagram given, which
Q37: A liquid has a molar heat of
Q109: Which of the following statements about viscosity