Multiple Choice
CO2 and H2 are allowed to react until an equilibrium is established as follows: 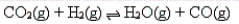 What will be the effect on the equilibrium of removing CO from the equilibrium mixture?
What will be the effect on the equilibrium of removing CO from the equilibrium mixture?
A) The equilibrium will favor the reactants side.
B) H2 concentration will double
C) CO and CO2 concentrations will double
D) H2 concentration will decrease and H2O concentration will increase
Correct Answer:

Verified
Correct Answer:
Verified
Q36: According to Le Chatelier's principle, which of
Q37: The minimum combined kinetic energy reactant particles
Q38: Consider the following equilibrim: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5982/.jpg" alt="Consider
Q39: Consider the following equilibrim: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5982/.jpg" alt="Consider
Q40: Which of the following statements concerning redox
Q42: For the indicated element, select the correct
Q43: In the redox reaction 2MnBr<sub>3</sub> + SnBr<sub>2</sub>
Q44: Which element is oxidized in the following
Q45: Which of the following statements about activation
Q46: For which of the following equilibrium systems