Multiple Choice
According to Le Chatelier's principle, which of the following changes will shift the position of the equilibrium to the left for the reaction 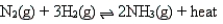
A) double the concentration of N2
B) decrease the concentration of NH3
C) increase the concentration of H2
D) triple the temperature
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Use the following to answer the questions
Q32: Which of the following statements concerning oxidation
Q33: Consider the following redox equation: SO<sub>2</sub> +
Q34: Assign the following reaction to one of
Q35: In writing an equilibrium constant expression, which
Q37: The minimum combined kinetic energy reactant particles
Q38: Consider the following equilibrim: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5982/.jpg" alt="Consider
Q39: Consider the following equilibrim: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5982/.jpg" alt="Consider
Q40: Which of the following statements concerning redox
Q41: CO<sub>2</sub> and H<sub>2</sub> are allowed to react