True/False
Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 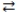 COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
COCl2(g). If chlorine gas is added to the reaction vessel, the concentration of carbon monoxide will decrease.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q13: The stronger the bond,the higher its bond
Q14: For an endothermic reaction at equilibrium,increasing the
Q41: Which of the following energy quantities is
Q42: Consider the following reversible reaction at equilibrium:
Q43: The rate of a chemical reaction increases
Q46: Which bond is the strongest?<br>A)H-Br<br>B)H-Cl<br>C)H-F<br>D)H-I
Q51: Consider the following reversible reaction at
Q58: In an _ reaction,the products are lower
Q85: Changes in potential energy occur in chemical
Q92: When reactants can come together and form