True/False
Consider the following reversible reaction at equilibrium: CO(g)+ Cl2(g) 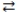 COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
COCl2(g). If the pressure inside the reaction vessel is increased, the equilibrium will shift to the left.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: The stronger the bond,the higher its bond
Q18: Exothermic reactions involve the formation of products
Q37: Consider the reaction: N<sub>2</sub>(g)+ O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5866/.jpg"
Q40: Increasing the temperature of a reaction mixture
Q41: Which of the following energy quantities is
Q43: The rate of a chemical reaction increases
Q46: Consider the following reversible reaction at equilibrium:
Q58: In an _ reaction,the products are lower
Q92: When reactants can come together and form
Q95: Le Châtelier's principle is a general rule