Multiple Choice
Estimate the bond angles around the sulfur atom in the structure shown below. 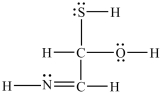
A) 90°
B) 109.5°
C) 120°
D) 180°
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: A double bond consists of four electrons
Q13: Every atom must have an octet of
Q15: Ethane (C<sub>2</sub>H<sub>6</sub>)is a polar molecule.
Q20: Phosphorus usually forms two covalent bonds in
Q35: Some covalent compounds are solids,some are liquids,and
Q54: Rank the atoms Br,Cl,and K in order
Q65: The shape around each carbon atom in
Q68: Which is the correct Lewis structure for
Q71: Which compound has the greatest number of
Q73: Which atom(s)in the structure below has(have)a