Solved
Which Atom(s)in the Structure Below Has(have)a Partial Negative Charge -)?
A)carbon
B)fluorine
C)hydrogen
D)nitrogen
E)nitrogen and Fluorine
Multiple Choice
Which atom(s) in the structure below has(have) a partial negative charge ( -) ? 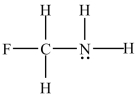
A) carbon
B) fluorine
C) hydrogen
D) nitrogen
E) nitrogen and fluorine
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: A double bond consists of four electrons
Q13: Every atom must have an octet of
Q35: Some covalent compounds are solids,some are liquids,and
Q54: Rank the atoms Br,Cl,and K in order
Q68: Which is the correct Lewis structure for
Q70: Estimate the bond angles around the sulfur
Q71: Which compound has the greatest number of
Q76: Aspartic acid is an amino acid used
Q77: The Lewis structure of formaldehyde is shown
Q78: The Lewis structure for the molecule below