Multiple Choice
Based on the functional group present, the compound below is classified as what type of compound? 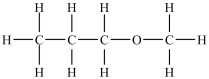
A) aldehyde
B) carboxylic acid
C) ether
D) ester
E) ketone
Correct Answer:

Verified
Correct Answer:
Verified
Q7: All molecules with polar bonds are polar
Q67: Alkanes,which have no functional groups,and therefore no
Q76: MTBE is soluble in both gasoline and
Q87: The compound below is a nonpolar molecule.
Q88: The compound below is an example of
Q89: The compound below is an example of
Q93: Which functional group is NOT present in
Q96: The molecule below is an example of
Q97: In order to complete the structure below,
Q111: Which compound has the highest boiling point?<br>A)HOCH<sub>2</sub>CH<sub>2</sub>OH<br>B)CH<sub>3</sub>NHCH<sub>2</sub>CH<sub>3</sub><br>C)CH<sub>3</sub>CO<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>D)NaCH<sub>3</sub>COO