True/False
The molecule below is an example of an aromatic compound. 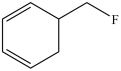
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: All molecules with polar bonds are polar
Q76: MTBE is soluble in both gasoline and
Q92: Based on the functional group present, the
Q93: Which functional group is NOT present in
Q97: In order to complete the structure below,
Q98: Which of the following correctly depicts the
Q99: The molecule below is an example of
Q100: Vitamin C is a water-soluble vitamin. <img
Q101: The two structures shown below represent the
Q111: Which compound has the highest boiling point?<br>A)HOCH<sub>2</sub>CH<sub>2</sub>OH<br>B)CH<sub>3</sub>NHCH<sub>2</sub>CH<sub>3</sub><br>C)CH<sub>3</sub>CO<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>D)NaCH<sub>3</sub>COO