Multiple Choice
Given the following values of equilibrium constants: 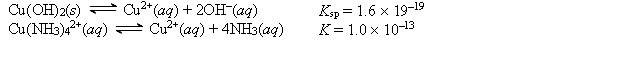 What is the value of the equilibrium constant for the following reaction? Cu(OH) 2(s) + 4NH3(aq)
What is the value of the equilibrium constant for the following reaction? Cu(OH) 2(s) + 4NH3(aq)  Cu(NH3) 42+(aq) + 2OH-(aq)
Cu(NH3) 42+(aq) + 2OH-(aq)
A) 1.6 10-19
B) 1.6 10-6
C) 6.2 1031
D) 1.0 1013
E) 1.6 10-32
Correct Answer:

Verified
Correct Answer:
Verified
Q62: You are given a solution of the
Q91: A 10-mL sample of tartaric acid is
Q154: The value of K<sub>sp</sub> for AgI is
Q155: You have 0.20 M HNO<sub>2</sub> (K<sub>a</sub> =
Q157: Calculate the pH when 200.0 mL of
Q158: Calculate the pH when 200.0 mL of
Q160: Solubility Products (K<sub>sp</sub>) <br>BaSO<sub>4</sub> 1.5 <font face="symbol"></font>
Q161: Calculate the pH when 200.0 mL of
Q162: A 50.0-mL sample of 2.0 <font face="symbol"></font>
Q164: A titration of 100.0 mL of 1.00