Multiple Choice
A 50.0-mL sample of 2.0 10-4 M CuNO3 is added to 50.0 mL of 4.0 M NaCN. Cu+ reacts with CN- to form the complex ion Cu(CN) 32-: 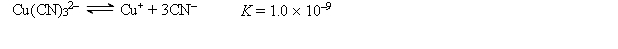 The concentration of Cu+ at equilibrium is
The concentration of Cu+ at equilibrium is
A) 5.0 10-14 M.
B) 1.2 10-14 M.
C) 2.0 10-4 M.
D) 1.0 10-4 M.
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q91: A 10-mL sample of tartaric acid is
Q157: Calculate the pH when 200.0 mL of
Q158: Calculate the pH when 200.0 mL of
Q159: Given the following values of equilibrium constants:
Q160: Solubility Products (K<sub>sp</sub>) <br>BaSO<sub>4</sub> 1.5 <font face="symbol"></font>
Q161: Calculate the pH when 200.0 mL of
Q164: A titration of 100.0 mL of 1.00
Q165: Calculate the pH when 200.0 mL of
Q166: If 22 mL of 0.50 M HCl
Q167: Which of the following compounds has the