Multiple Choice
Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 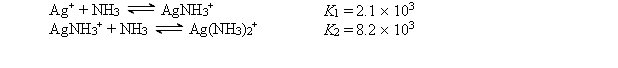 The concentration of Ag+ at equilibrium is
The concentration of Ag+ at equilibrium is
A) 2.0 M.
B) 4.5 10-9 M.
C) 1.2 10-8 M.
D) 1.6 M.
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q68: How many mmoles of HCl must be
Q70: Which titration curve would result from the
Q71: The overall K<sub>f</sub> for the complex ion
Q72: A 200.0-mL sample of the weak acid
Q74: Calculate the molar solubility of BaCO<sub>3</sub> (K<sub>sp</sub>
Q75: For carbonic acid (H<sub>2</sub>CO<sub>3</sub>), K<sub>a1</sub> = 4.30
Q76: A 200.0-mL sample of the weak acid
Q77: The contents of the flask are transferred
Q77: An indicator HIn has K<sub>a</sub> = 1
Q78: If 30 mL of 5.0 <font face="symbol"></font>