Multiple Choice
Which titration curve would result from the titration of a strong base by a strong monoprotic acid?
A) 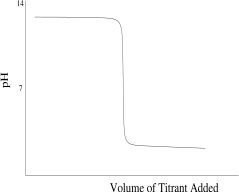
B) 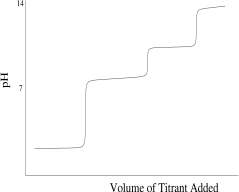
C) 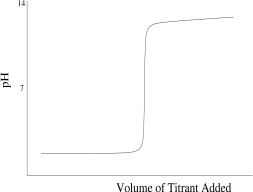
D) 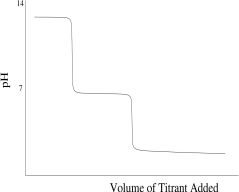
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q45: What is the pH of this solution?<br>A)
Q68: How many mmoles of HCl must be
Q71: The overall K<sub>f</sub> for the complex ion
Q72: A 200.0-mL sample of the weak acid
Q73: Consider a solution made by mixing 500.0
Q74: Calculate the molar solubility of BaCO<sub>3</sub> (K<sub>sp</sub>
Q75: For carbonic acid (H<sub>2</sub>CO<sub>3</sub>), K<sub>a1</sub> = 4.30
Q77: The contents of the flask are transferred
Q90: The pH at the equivalence point of
Q158: Buffers in the human body<br>A) precipitate proteins