Multiple Choice
The cation M2+ reacts with NH3 to form a series of complex ions as follows: 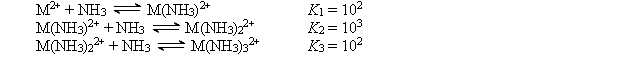 A 1.0 10-3 mol sample of M(NO3) 2 is added to 1.0 L of 15.0 M NH3 (Kb = 1.8 10-5) . Which of the following would be a dominant species in this solution?
A 1.0 10-3 mol sample of M(NO3) 2 is added to 1.0 L of 15.0 M NH3 (Kb = 1.8 10-5) . Which of the following would be a dominant species in this solution?
A) M(NH3) 2+
B) M(NH3) 32+
C) M2+
D) M(NH3) 22+
Correct Answer:

Verified
Correct Answer:
Verified
Q45: What is the pH of this solution?<br>A)
Q59: A 50.0-mL solution of the acid H<sub>3</sub>A
Q60: The Ag<sup>+</sup> ion reacts with NH<sub>3</sub> to
Q61: Calculate the pH of a solution made
Q62: Consider the titration of 100.0 mL of
Q63: For the compound MX, K<sub>sp</sub> is 2.00
Q68: How many mmoles of HCl must be
Q77: The contents of the flask are transferred
Q90: The pH at the equivalence point of
Q158: Buffers in the human body<br>A) precipitate proteins