Essay
The Ag+ ion reacts with NH3 to form the following complex ions: 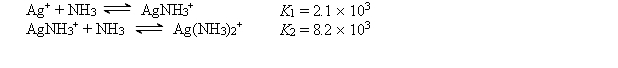 AgCl (Ksp = 1.6 *10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
AgCl (Ksp = 1.6 *10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
Correct Answer:

Verified
[AG+] = 3.3 *10View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q55: In the titration of a weak acid
Q57: Calculate the solubility of Ag<sub>2</sub>CrO<sub>4</sub> [K<sub>sp</sub> =
Q58: The K<sub>sp</sub> for Mn(OH)<sub>2</sub> is 2.0 <font
Q59: A 50.0-mL solution of the acid H<sub>3</sub>A
Q61: Calculate the pH of a solution made
Q62: Consider the titration of 100.0 mL of
Q63: For the compound MX, K<sub>sp</sub> is 2.00
Q64: The cation M<sup>2+</sup> reacts with NH<sub>3</sub> to
Q135: Consider the following information about the diprotic
Q158: Buffers in the human body<br>A) precipitate proteins