Multiple Choice
Given the following bond energies: 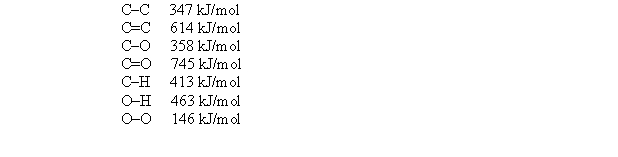 estimate H for the reaction H2O2 + CH3OH H2CO + 2H2O.
estimate H for the reaction H2O2 + CH3OH H2CO + 2H2O.
A) -145 kJ
B) +291 kJ
C) -291 kJ
D) +145 kJ
E) -287 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Using the following data reactions:
Q4: Given the following information: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6420/.jpg"
Q5: The Lewis structure for H<sub>3</sub>BO<sub>3</sub> is<br>A) <img
Q6: Of the following, which molecule has the
Q8: When molten sulfur reacts with chlorine gas,
Q10: Which ion is planar?<br>A) CO<sub>3</sub><sup>2</sup><sup>-</sup><br>B) SO<sub>4</sub><sup>2</sup><sup>-</sup><br>C) PCl<sub>4</sub><sup>+</sup><br>D)
Q11: The molecule XCl<sub>5</sub><sup>-</sup><sup> </sup>has a square pyramidal
Q48: Choose the molecule with the strongest bond.<br>A)
Q55: Based on electronegativities, which of the following
Q56: Which molecule or ion violates the octet