Multiple Choice
When molten sulfur reacts with chlorine gas, a vile-smelling orange liquid forms that is found to have the empirical formula SCl. Which of the following could be the correct Lewis structure for this compound?
A) 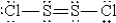
B) 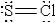
C) 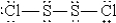
D) 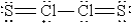
E) 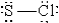
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Given the following bond energies: <img
Q4: Given the following information: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6420/.jpg"
Q5: The Lewis structure for H<sub>3</sub>BO<sub>3</sub> is<br>A) <img
Q6: Of the following, which molecule has the
Q10: Which ion is planar?<br>A) CO<sub>3</sub><sup>2</sup><sup>-</sup><br>B) SO<sub>4</sub><sup>2</sup><sup>-</sup><br>C) PCl<sub>4</sub><sup>+</sup><br>D)
Q11: The molecule XCl<sub>5</sub><sup>-</sup><sup> </sup>has a square pyramidal
Q13: Of the following, which molecule has the
Q48: Choose the molecule with the strongest bond.<br>A)
Q56: Which molecule or ion violates the octet
Q100: Which of the following statements is incorrect?<br>A)