Multiple Choice
Aluminum metal crystallizes in a face-centered cubic structure. What is the relationship between the radius of an Al atom (r) and the length of an edge of the unit cell (E) ?
A) 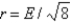
B) 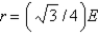
C) r = 2E
D) r = 4E
E) r = E/2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Which intermolecular force is the strongest?<br>A) polar
Q20: Which of the following statements is/are true
Q21: The molar volume of a certain form
Q22: Which of the following chemical species has
Q23: You are given the following boiling-point data:
Q25: On the basis of your knowledge of
Q26: Which of the compounds below is an
Q27: A certain substance has the phase diagram
Q28: The structure for CaF<sub>2</sub> can be described
Q29: Which of the following statements is incorrect?<br>A)