Multiple Choice
A certain substance has the phase diagram shown below. At which of the following values of T and P is the substance a pure liquid? 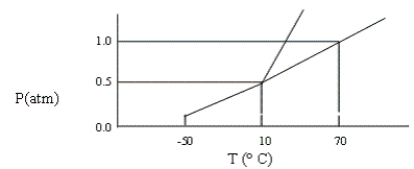
A) T = 70°C, P = 1.2 atm
B) T = 10°C, P = 1 atm
C) T = 8°C, P = 1 atm
D) T = 10°C, P = 0.5 atm
E) T = 80°C, P = 1 atm
Correct Answer:

Verified
Correct Answer:
Verified
Q22: Which of the following chemical species has
Q23: You are given the following boiling-point data:
Q24: Aluminum metal crystallizes in a face-centered cubic
Q25: On the basis of your knowledge of
Q26: Which of the compounds below is an
Q28: The structure for CaF<sub>2</sub> can be described
Q29: Which of the following statements is incorrect?<br>A)
Q30: Sodium oxide (Na<sub>2</sub>O) crystallizes in a structure
Q31: Based on intermolecular forces, which of the
Q32: Which substance can be described as cations