Multiple Choice
Pure rubidium crystallizes in a body-centered cubic lattice; the edge length of the unit cell is 562 pm. What is the density of rubidium in grams per cubic centimeter?
A) 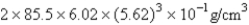
B) 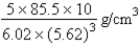
C) 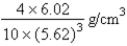
D) 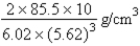
E) 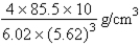
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: Which of the following statements is true
Q50: KCl crystallizes in a structure like NaCl.
Q51: A certain solid substance that is very
Q52: The heat of vaporization of a certain
Q53: The normal boiling point of liquid X
Q55: Metallic copper crystallizes in a face-centered cubic
Q56: When 1.00 mol of a pure liquid
Q57: Given the graph below, what is the
Q58: A salt, MY, crystallizes in a body-centered
Q59: Knowing that ΔH<sub>vap</sub> for water is 40.7