Multiple Choice
Given the graph below, what is the boiling point of carbon tetrachloride at standard pressure? 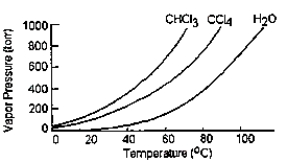
A) The graph does not give this information.
B) 98°C
C) 77°C
D) 60°C
E) 34°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: The heat of vaporization of a certain
Q53: The normal boiling point of liquid X
Q54: Pure rubidium crystallizes in a body-centered cubic
Q55: Metallic copper crystallizes in a face-centered cubic
Q56: When 1.00 mol of a pure liquid
Q58: A salt, MY, crystallizes in a body-centered
Q59: Knowing that ΔH<sub>vap</sub> for water is 40.7
Q60: The table below lists the ionic radii
Q61: Which one of the following is the
Q62: Choose the correct statement about the diagram