Essay
Pure N2O3 was placed in a vessel and allowed to decompose:
2N2O3(g) → 2N2(g) + 3O2(g)
The following data were collected in an experiment at a temperature at which 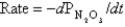
. 
A. Using these data, construct the appropriate plot(s), and determine the differential rate law.
B. Calculate the value of the rate constant k at this temperature.
C. Give the values of the first, second, and fifth half-lives.
D. Calculate the total pressure expected at t = 35 min.
Correct Answer:

Verified
A. 
B. 7.5 × 10-3S1U1...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
B. 7.5 × 10-3S1U1...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q23: Which of the following will a catalyst
Q24: The following questions refer to the reaction
Q25: The following questions refer to the gas-phase
Q26: Svante Arrhenius proposed the existence of threshold
Q27: The decomposition of N<sub>2</sub>O<sub>5</sub>(g) to NO<sub>2</sub>(g) and
Q29: The elementary chemical reaction<br>O + ClO →
Q30: The decomposition of N<sub>2</sub>O<sub>5</sub>(g) to NO<sub>2</sub>(g) and
Q31: Is this reaction exothermic or endothermic? <br>A)
Q32: The oxidation of Cr<sup>3+</sup> to CrO<sub>4</sub><sup>2-</sup> can
Q33: Consider the reaction<br>3A + B + C