Multiple Choice
The following questions refer to the reaction 2A2 + B2 → 2C. The mechanism below has been proposed: 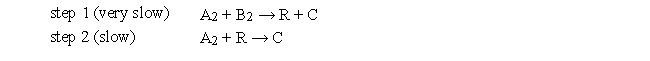
-When ethyl chloride, CH3CH2Cl, is dissolved in 1.0 M NaOH, it is converted into ethanol, CH3CH2OH, by the reaction
CH3CH2Cl + OH- → CH3CH2OH + Cl-
At 25°C the reaction is first order in CH3CH2Cl, and the rate constant is 1.0 × 10-3 s-1. If the activation parameters are A = 3.4 × 1014 s-1 and Ea = 100.0 kJ/mol, what will the rate constant be at 36°C? (R = 8.314 J/mol • K)
A) 4.9× 1014 s-1
B) 1.4× 10-3 s-1
C) 6.9× 102 s-1
D) 9.3× 10-3 s-1
E) 4.2× 10-3 s-1
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Tabulated below are initial rate data for
Q20: The following initial rate data were found
Q22: The OH radical disproportionates according to the
Q23: Which of the following will a catalyst
Q25: The following questions refer to the gas-phase
Q26: Svante Arrhenius proposed the existence of threshold
Q27: The decomposition of N<sub>2</sub>O<sub>5</sub>(g) to NO<sub>2</sub>(g) and
Q28: Pure N<sub>2</sub>O<sub>3</sub> was placed in a vessel
Q29: The elementary chemical reaction<br>O + ClO →
Q66: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="For the