Multiple Choice
A mixture of hydrogen and chlorine remains unreacted until it is exposed to ultraviolet light from a burning magnesium strip. Then the following reaction occurs very rapidly. 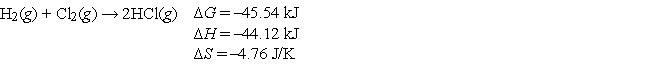 Select the statement below that best explains this behavior.
Select the statement below that best explains this behavior.
A) The reactants are thermodynamically more stable than the products.
B) The reaction is spontaneous, but the reactants are kinetically stable.
C) The reaction has a small equilibrium constant.
D) The ultraviolet light raises the temperature of the system and makes the reaction more favorable.
E) The negative value for ΔS slows down the reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q113: One mole of an ideal gas at
Q114: For a spontaneous endothermic process, which conditions
Q115: The process H<sub>2</sub>O(g) → H<sub>2</sub>O(l) takes place
Q116: The process H<sub>2</sub>O(g) → H<sub>2</sub>O(l) takes place
Q117: Consider a weak acid, HX. The value
Q119: The reaction<br>2H<sub>2</sub>O(g) → 2H<sub>2</sub>(g) + O<sub>2</sub>(g)<br>Has a
Q120: The vapor pressure of Br<sub>2</sub>(l) at 25°C
Q121: In the equation P<sub>1</sub>V<sub>1</sub><br><sub> </sub> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg"
Q122: Consider the reaction below at 25°C, for
Q123: Consider the gas phase reaction<br>NO + (1/2)O<sub>2</sub>