Essay
Consider the reaction below at 25°C, for which the following data are relevant. 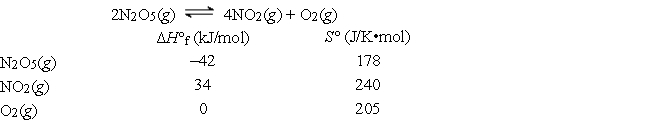
Calculate ΔH°, ΔS°, and ΔG° for this reaction at 25°C.
Correct Answer:

Verified
ΔH° = 220....View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q117: Consider a weak acid, HX. The value
Q118: A mixture of hydrogen and chlorine remains
Q119: The reaction<br>2H<sub>2</sub>O(g) → 2H<sub>2</sub>(g) + O<sub>2</sub>(g)<br>Has a
Q120: The vapor pressure of Br<sub>2</sub>(l) at 25°C
Q121: In the equation P<sub>1</sub>V<sub>1</sub><br><sub> </sub> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg"
Q123: Consider the gas phase reaction<br>NO + (1/2)O<sub>2</sub>
Q124: One mole of an ideal gas expands
Q125: For the reaction 2HF(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="For
Q126: For the process involving compound A: A(s)
Q127: Consider the following hypothetical reaction (at 307