Multiple Choice
Given the following data, calculate the normal boiling point for formic acid (HCOOH) . 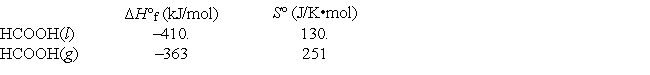
A) 115°C
B) 82°C
C) 2.57 K
D) 1730°C
E) 388°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q123: Consider the gas phase reaction<br>NO + (1/2)O<sub>2</sub>
Q124: One mole of an ideal gas expands
Q125: For the reaction 2HF(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="For
Q126: For the process involving compound A: A(s)
Q127: Consider the following hypothetical reaction (at 307
Q129: Water gas, a commercial fuel, is made
Q130: The equilibrium constant K<sub>p</sub> for the dissociation
Q131: One mole of an ideal gas is
Q132: For which process is ΔS negative?<br>A) evaporation
Q133: The standard free energy of formation of