Multiple Choice
Consider the following hypothetical reaction (at 307 K) . Standard free energies, in kJ/mol, are given in parentheses. 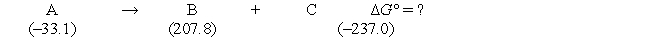 What is the value of the equilibrium constant for the reaction at 307 K?
What is the value of the equilibrium constant for the reaction at 307 K?
A) 1.5
B) 0.22
C) 1.0
D) 3.9 × 1010
E) 0.18
Correct Answer:

Verified
Correct Answer:
Verified
Q122: Consider the reaction below at 25°C, for
Q123: Consider the gas phase reaction<br>NO + (1/2)O<sub>2</sub>
Q124: One mole of an ideal gas expands
Q125: For the reaction 2HF(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="For
Q126: For the process involving compound A: A(s)
Q128: Given the following data, calculate the normal
Q129: Water gas, a commercial fuel, is made
Q130: The equilibrium constant K<sub>p</sub> for the dissociation
Q131: One mole of an ideal gas is
Q132: For which process is ΔS negative?<br>A) evaporation