Multiple Choice
At room temperature cyclohexane exists almost exclusively in the chair conformation (99.99%) . But at 800°C, 30% of the cyclohexane molecules exist in the twist-boat conformation.What is the value of the equilibrium constant for the following reaction at 800°C?
C6H12(chair) 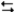 C6H12(twist-boat)
C6H12(twist-boat)
A) 0.23
B) 0.30
C) 2.3
D) 0.43
E) 0.77
Correct Answer:

Verified
Correct Answer:
Verified
Q80: A gas expands isothermally and irreversibly.<br>-ΔS<sub>surr</sub> is<br>A)
Q81: The standard free energy of formation of
Q82: Consider the reaction<br>2N<sub>2</sub>O<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider the
Q83: Consider the following system at equilibrium at
Q84: Substance X has a heat of vaporization
Q86: Which of the following statements is/are true
Q87: A gas phase decomposition reaction<br>2AB<sub>3</sub> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg"
Q88: For the process moleene(l) → moleene(g) at
Q89: One mole of an ideal gas at
Q90: At 1 atm, a liquid is heated