Multiple Choice
A gas phase decomposition reaction
2AB3 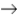 A2 + 3B2
A2 + 3B2
Is exothermic. What can you conclude about the spontaneity of this reaction?
A) It is non-spontaneous.
B) Nothing conclusive can be stated without more information.
C) It is spontaneous.
Correct Answer:

Verified
Correct Answer:
Verified
Q82: Consider the reaction<br>2N<sub>2</sub>O<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider the
Q83: Consider the following system at equilibrium at
Q84: Substance X has a heat of vaporization
Q85: At room temperature cyclohexane exists almost exclusively
Q86: Which of the following statements is/are true
Q88: For the process moleene(l) → moleene(g) at
Q89: One mole of an ideal gas at
Q90: At 1 atm, a liquid is heated
Q91: In a certain reversible expansion, a system
Q92: For this system at equilibrium, how will