Multiple Choice
A certain chemical reaction sets up an equilibrium as follows
3A2(aq) + 2B3(aq) 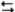 6AB(aq)
6AB(aq)
After being started with unknown amounts of A2 and B3. Once the system reaches equilibrium the concentrations of A2, B3, and AB are precisely determined and the temperature recorded. What other quantities can be determined? Q 0 is the reaction quotient at the initial conditions.
A) Q 0, Kc, K, ΔG
B) Q 0, K, ΔG, ΔG°
C) K, Kc, ΔG, ΔG°
D) Q 0, Kc, ΔG, ΔG°
E) Q 0, Kc, K, ΔG°
Correct Answer:

Verified
Correct Answer:
Verified
Q133: The standard free energy of formation of
Q134: Substance X has a heat of vaporization
Q135: For the reaction A + B →
Q136: Choose the correct statement.<br>A) A reaction that
Q137: A gas expands isothermally and irreversibly.<br>-q is<br>A)
Q138: In an isothermal process, the pressure on
Q139: For the process A(l) → A(g), ΔH°
Q140: Given that ΔG°<sub>f</sub> for NH<sub>3</sub> = -16.67
Q141: The vapor pressure of Br<sub>2</sub>(l) at 25°C
Q142: The equilibrium constant K<sub>p</sub> (in atm) for