Multiple Choice
Consider the following information about the diprotic acid ascorbic acid (H2As for short, molar mass = 176.1) .  The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below:
The titration curve for disodium ascorbate, Na2As, with standard HCl is shown below: 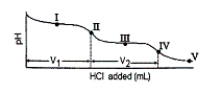
-What major species is(are) present at point III?
A) HAs- only
B) H2As and H+
C) H2As only
D) As2- and HAs-
E) HAs- and H2As
Correct Answer:

Verified
Correct Answer:
Verified
Q16: Derive the Henderson-Hasselbalch equation from the K<sub>a</sub>
Q17: Silver acetate (AgC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>) is a sparingly soluble
Q18: What is the pH of a solution
Q19: A 200.0-mL sample of the weak acid
Q20: You dissolve 1.22 g of an unknown
Q22: You have two salts, AgX and AgY,
Q23: Given the following values of equilibrium constants:
Q24: Sodium chloride is added slowly to a
Q25: What quantity of NaOH(s) must be added
Q26: Methyl orange is an indicator with a