Multiple Choice
Given the following values of equilibrium constants: 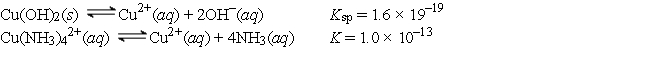 What is the value of the equilibrium constant for the following reaction?
What is the value of the equilibrium constant for the following reaction?
Cu(OH) 2(s) + 4NH3(aq) 
Cu(NH3) 42+(aq) + 2OH-(aq)
A) 1.6 × 10-19
B) 1.6 × 10-6
C) 6.2 × 1031
D) 1.0 × 1013
E) 1.6 × 10-32
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: What is the pH of a solution
Q19: A 200.0-mL sample of the weak acid
Q20: You dissolve 1.22 g of an unknown
Q21: Consider the following information about the diprotic
Q22: You have two salts, AgX and AgY,
Q24: Sodium chloride is added slowly to a
Q25: What quantity of NaOH(s) must be added
Q26: Methyl orange is an indicator with a
Q27: A solution containing 10. mmol of CO<sub>3</sub><sup>2-</sup>
Q28: Equal volumes of 0.1 M HCl and