Multiple Choice
You have a solution of 0.10 M Cl- and 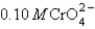 . You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 × 10-12 and for AgCl is1.6 × 10-10. Which of the following will precipitate first?
. You add 0.10 M silver nitrate dropwise into the solution. Ksp for Ag2CrO4 is 9.0 × 10-12 and for AgCl is1.6 × 10-10. Which of the following will precipitate first?
A) silver nitrate
B) silver chromate
C) cannot be determined from the information given
D) silver chloride
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q94: How many mmoles of HCl must be
Q95: The solubility of AgCl in water is
Q96: A 5.95-g sample of an acid, H<sub>2</sub>X,
Q97: How many moles of Ca(NO<sub>3</sub>)<sub>2</sub> must be
Q98: A certain indicator HIn has a pK<sub>a</sub>
Q100: A solution containing 10. mmol of CO<sub>3</sub><sup>2-</sup>
Q101: A solution is prepared by mixing hydrazoic
Q102: Calculate the pH when 200.0 mL of
Q103: Which of the following titration curves schematically
Q104: Calculate the minimum concentration of 100.0 mL