Multiple Choice
Which of the following titration curves schematically represents a diprotic acid being titrated by a strong base?
A) 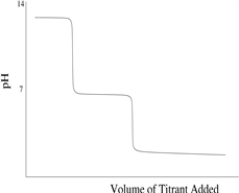
B) 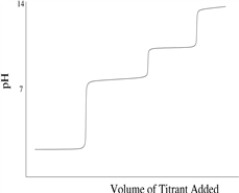
C) 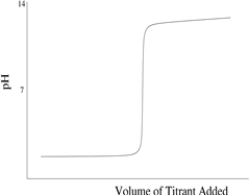
D) 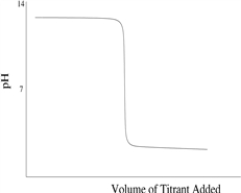
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q98: A certain indicator HIn has a pK<sub>a</sub>
Q99: You have a solution of 0.10 M
Q100: A solution containing 10. mmol of CO<sub>3</sub><sup>2-</sup>
Q101: A solution is prepared by mixing hydrazoic
Q102: Calculate the pH when 200.0 mL of
Q104: Calculate the minimum concentration of 100.0 mL
Q105: Calculate the concentration of Ag<sup>+</sup> in a
Q106: Differentiate between a formation constant and a
Q107: A 56.20-mL sample of 0.0782 M HCN
Q108: If 16 mL of 0.78 M HCl