Multiple Choice
Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 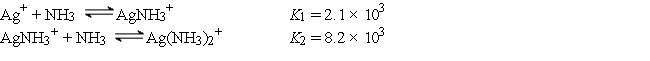 The concentration of Ag(NH3) 2+ at equilibrium is
The concentration of Ag(NH3) 2+ at equilibrium is
A) 0.20 M.
B) 2.0 M.
C) 0.40 M.
D) 1.0 × 10-3 M.
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q83: Derive the equation describing the relationship between
Q84: An indicator HIn has K<sub>a</sub> = 1
Q85: Which of the following statements is/are true
Q86: Calculate [H<sup>+</sup>] in a solution that is
Q87: A solution is formed by mixing 50.0
Q89: 12.4 mL of 0.25 M HCl is
Q90: The pH at the equivalence point of
Q91: A 10-mL sample of tartaric acid is
Q92: For the compound MX, K<sub>sp</sub> is 2.00
Q93: Consider the titration of 200.0 mL of