Essay
The cation M2+ reacts with NH3 to form a series of complex ions as follows: 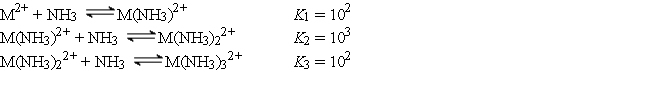
Consider an experiment in which 1.0 × 10-3 mol of M(NO3)2 is added to 1.0 L of 15.0 M NH3. Calculate the equilibrium concentrations of M2+, M(NH3)22+, and M(NH3)32+
Correct Answer:

Verified
[M2+] = 3.0 × 10-14 M; [M...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q55: What is the solubility of Mg(OH)<sub>2</sub> (K<sub>sp</sub>
Q56: Calculate the pH of a solution made
Q57: A 50.0-mL sample of 2.0 × 10<sup>-4</sup>
Q58: Chromate ion is added to a saturated
Q59: A student uses 16.60 mL of 0.100
Q61: What is the molarity of a sodium
Q62: You are given a solution of the
Q63: A titration of 100.0 mL of 1.00
Q64: Calculate the pH when 200.0 mL of
Q65: Consider the titration of 100.0 mL of