Multiple Choice
The magnetic quantum number 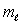 is most closely associated with what property of the electron in an atom?
is most closely associated with what property of the electron in an atom?
A) Magnitude of the orbital angular momentum
B) Energy
C) z component of the spin angular momentum
D) z component of the orbital angular momentum
E) Radius of the orbit
Correct Answer:

Verified
Correct Answer:
Verified
Q12: The effective charge acting on a single
Q12: An electron in a K shell
Q16: The number of possible values of the
Q18: A magnetic dipole <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3150/.jpg" alt="A magnetic
Q20: The K <sub> <span class="ql-formula" data-value="\infty"><span
Q30: Characteristic K x-radiation of an element is
Q36: Which of the following subshells cannot exist?<br>A)3p<br>B)2p<br>C)4d<br>D)3d<br>E)2d
Q49: The Pauli exclusion principle is obeyed by:<br>A)all
Q51: Two different electron beams are incident on
Q78: The "e" in laser stands for:<br>A)electric<br>B)emf<br>C)energy<br>D)emission<br>E)entropy