Multiple Choice
The number of possible values of the magnetic quantum number 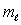 associated with a given value of the orbital quantum number
associated with a given value of the orbital quantum number 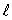 is:
is:
A) 1
B) 2
C) 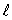
D) 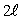
E) 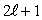
Correct Answer:

Verified
Correct Answer:
Verified
Q12: The effective charge acting on a single
Q12: An electron in a K shell
Q15: The magnetic quantum number <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3150/.jpg" alt="The
Q18: A magnetic dipole <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3150/.jpg" alt="A magnetic
Q20: The K <sub> <span class="ql-formula" data-value="\infty"><span
Q21: The number of values of the orbital
Q36: Which of the following subshells cannot exist?<br>A)3p<br>B)2p<br>C)4d<br>D)3d<br>E)2d
Q49: The Pauli exclusion principle is obeyed by:<br>A)all
Q51: Two different electron beams are incident on
Q78: The "e" in laser stands for:<br>A)electric<br>B)emf<br>C)energy<br>D)emission<br>E)entropy