Multiple Choice
The number of states in a subshell with orbital quantum number 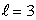 is:
is:
A) 2
B) 3
C) 7
D) 9
E) 14
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Which of the following is essential for
Q35: In connection with x-ray emission the
Q38: An electron in an L shell
Q52: An electron in an atom is in
Q53: The group of atoms at the beginning
Q55: The number of states in a shell
Q56: An electron is in a quantum state
Q58: The possible values for the magnetic
Q61: The total number of electron states with
Q66: For any atom other that hydrogen and