Multiple Choice
An electron in an atom is in a state with principal quantum number n = 4. The possible values of the orbital quantum number 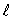 are:
are:
A) 1, 2, 3
B) 1, 2, 3, 4
C) -3, -2, -1, 0, 1, 2, 3
D) 0, 1, 2, 3
E) 0, 1, 2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Which of the following is essential for
Q47: The transition shown gives rise to
Q48: Five electrons are in a two-dimensional square
Q49: A magnetic dipole is placed between the
Q50: Suppose the energy required to ionize an
Q51: A group of electromagnetic waves might <img
Q53: The group of atoms at the beginning
Q55: The number of states in a shell
Q56: An electron is in a quantum state
Q57: The number of states in a subshell