Multiple Choice
If electrons did not have intrinsic angular momentum (spin) but still obeyed the Pauli exclusion principle the states occupied by electrons in the ground state of helium would be:
A) (n = 1, 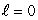 ) ; (n = 1,
) ; (n = 1, 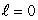 )
)
B) (n = 1, 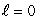 ) ; (n = 1,
) ; (n = 1, 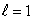 )
)
C) (n = 1, 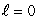 ) ; (n = 2,
) ; (n = 2,  )
)
D) (n = 2, 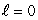 ) ; (n = 2,
) ; (n = 2, 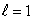 )
)
E) (n = 2, 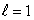 ) ; (n = 2,
) ; (n = 2, 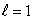 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Electrons in a certain laser make transitions
Q33: Possible values of the principal quantum
Q35: In the relation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3150/.jpg" alt="
Q36: In connection with x-ray emission the
Q37: The most energetic electron in any atom
Q39: An electron in an atom is
Q41: Six electrons are in a two-dimensional square
Q43: The most energetic electron in any atom
Q44: In a helium-neon laser, the laser light
Q57: The purpose of the mirrors at the